– Significant Improvements in Behavioral Symptoms Observed in Patients and Sustained through 38 Weeks of Treatment with ZYN002 –
– Presentation Today at the 16th NFXF International Fragile X Conference –
Devon, PA, July 12, 2018 — Zynerba Pharmaceuticals, Inc. (NASDAQ:ZYNE), the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders, is reporting today new open label clinical data in an oral presentation at the 16th NFXF (National Fragile X Foundation) International Fragile X Conference. The presentation will take place during the Industry Updates session at 4:30 PM EDT today in the Regency Ballroom of the Hyatt Regency, Cincinnati Ohio. A copy of the presentation and poster are available on the Zynerba corporate website at http://zynerba.com/publications/.
In a podium presentation entitled, “Transdermal Cannabidiol (CBD) Gel for the Treatment of Fragile X Syndrome (FXS),” Liza A. Squires, M.D., Zynerba’s Chief Medical Officer, will present new 12- and 38-week data describing significant and sustained improvements in behavioral symptoms with continued use of ZYN002 in children and adolescents with FXS. The presentation includes data through 38 weeks of treatment with ZYN002 in the open label Phase 2 FAB-C (Treatment of Fragile X Syndrome Anxiety and Behavioral Challenges with CBD) trial.
The data demonstrate that treatment with ZYN002 improved core behavioral symptoms of Fragile X syndrome with statistical significance versus baseline across multiple measures of efficacy at week 12, and these improvements were sustained through 38 weeks of treatment. ZYN002 was well tolerated; no serious adverse events were reported, and no clinically meaningful trends in vital signs, ECG, or clinical safety laboratories, including liver function tests (LFTs), were observed.
“These data are consistent and compelling, and suggest that ZYN002 may have a clinically meaningful and durable effect on the most common observable behaviors associated with childhood and adolescent Fragile X syndrome,” said Honey Heussler, FRACP, DM, Associate Professor, University of Queensland and Medical Director Child Development, Children’s Health Queensland, and lead investigator in the FAB-C study. “The goal of an ideal therapeutic intervention is to reduce the severity and impact of these core symptoms, and thus improve the child’s ability to engage with the world around them, including with their parents, caregivers, teachers and peers. It is very encouraging that measurable improvements in behaviors were observed in this study across a variety of instruments, whether validated for use by caregivers or physicians. I am delighted by these data; however, we need the results from the recently initiated CONNECT-FX study for confirmation. I look forward to participating in the continued development of ZYN002 for these children.”
Study design
Twenty patients (3:1 males) aged 6 to 17 years of age (median = 9) with Fragile X were enrolled in the open label FAB-C study. All patients had genetic confirmation of the full mutation of the FMR1 gene. ZYN002 was added to other medications being administered. The first six weeks were designed to titrate dosing in patients. Dosing was initiated at 50 mg daily and could be increased to 250 mg daily. Weeks seven through 12 was a maintenance period where patients were treated at the dose established at week six. Two patients, who were siblings, discontinued during the initial 12-week period; one discontinued due to worsening eczema (not considered treatment related) and the other discontinued for administrative reasons. At the completion of week 12, 13 patients elected to enter into the extension study for up to 24 months. To date, 12 patients remain in the study and have now exceeded 12 months of therapy with ZYN002.
Week 12 Efficacy
As previously disclosed, the 12-week FAB-C study achieved its primary endpoint, which was reduction in the Anxiety, Depression, and Mood Scale (ADAMS) total score from baseline to week 12 (n=18; 45.8% reduction; p<0.0001). Four of the five subscales of ADAMS also showed statistically significant reductions versus baseline.
| Scale: ADAMS |
Baseline
(n=20) |
Week 12
(n=18) |
Week 12
% Improvement Group Mean |
P-value vs Baseline |
| ADAMS Total Score |
33.4 |
18.1 |
45.8 |
< 0.0001 |
| General Anxiety |
10.0 |
4.6 |
54.0 |
< 0.0001 |
| Social Avoidance |
10.2 |
4.8 |
52.9 |
0.0002 |
| Compulsive Behavior |
2.8 |
1.4 |
50.0 |
0.0262 |
| Manic/Hyperactive Behavior |
9.4 |
6.1 |
35.1 |
0.0003 |
| Depressed Mood |
2.8 |
2.0 |
28.6 |
0.1417 |
As previously disclosed, ZYN002 achieved statistically significant reductions in all six subscales of a secondary endpoint, the Aberrant Behavior Checklist – Community: FXS Specific (ABC-CFXS).
| Scale: ABC-CFXS |
Baseline
(n=20) |
Week 12
(n=18) |
Week 12
% Improvement Group Mean |
P-value vs Baseline |
| Stereotypy |
7.9 |
3.2 |
59.5 |
0.0006 |
| Social Avoidance |
5.1 |
2.3 |
54.9 |
0.0005 |
| Socially Unresponsive/Lethargic |
8.7 |
4.1 |
52.9 |
0.0034 |
| Inappropriate Speech |
6.1 |
3.5 |
42.6 |
0.0018 |
| Irritability |
18.2 |
10.6 |
41.8 |
0.0096 |
| Hyperactivity |
14.5 |
9.8 |
32.4 |
0.0237 |
Zynerba is also releasing today the results of additional secondary efficacy endpoints that were evaluated in the first 12 weeks of the FAB-C trial, which the Company believes further reinforces the results from the ADAMS and ABC-CFXS , regardless of whether assessments were completed by a caregiver or physician. These measurements include:
| Scale |
Baseline
(n=20) |
Week 12
(n=18) |
Week 12
% Improvement Group Mean |
P-value vs Baseline |
| CGI-I |
n/a |
2.5* |
n/a |
n/a |
| PARS-R |
15.6 |
10.6 |
32.1 |
0.0006 |
| PedsQL: Total |
57.3 |
67.7 |
18.2 |
0.0100 |
| VAS: Hyperactivity/Impulsivity |
6.2 |
3.6 |
41.9 |
0.0002 |
| VAS: Tantrum/Mood Lability |
5.0 |
3.2 |
36.0 |
0.0023 |
| VAS: Anxiety |
6.2 |
3.8 |
38.7 |
0.0005 |
| Vineland Adaptive Behavior |
48.3 |
48.9 |
1.2 |
0.0472 |
* N=17 at week 12
The Clinical Global Impression Scale – Improvement (CGI-I) is a clinician-rated single-item 7-point scale that measures change in a patient’s condition following the start of a treatment. Ratings of one through three represent improvement; a rating of four represents no change; ratings of five through seven represent worsening of symptoms.
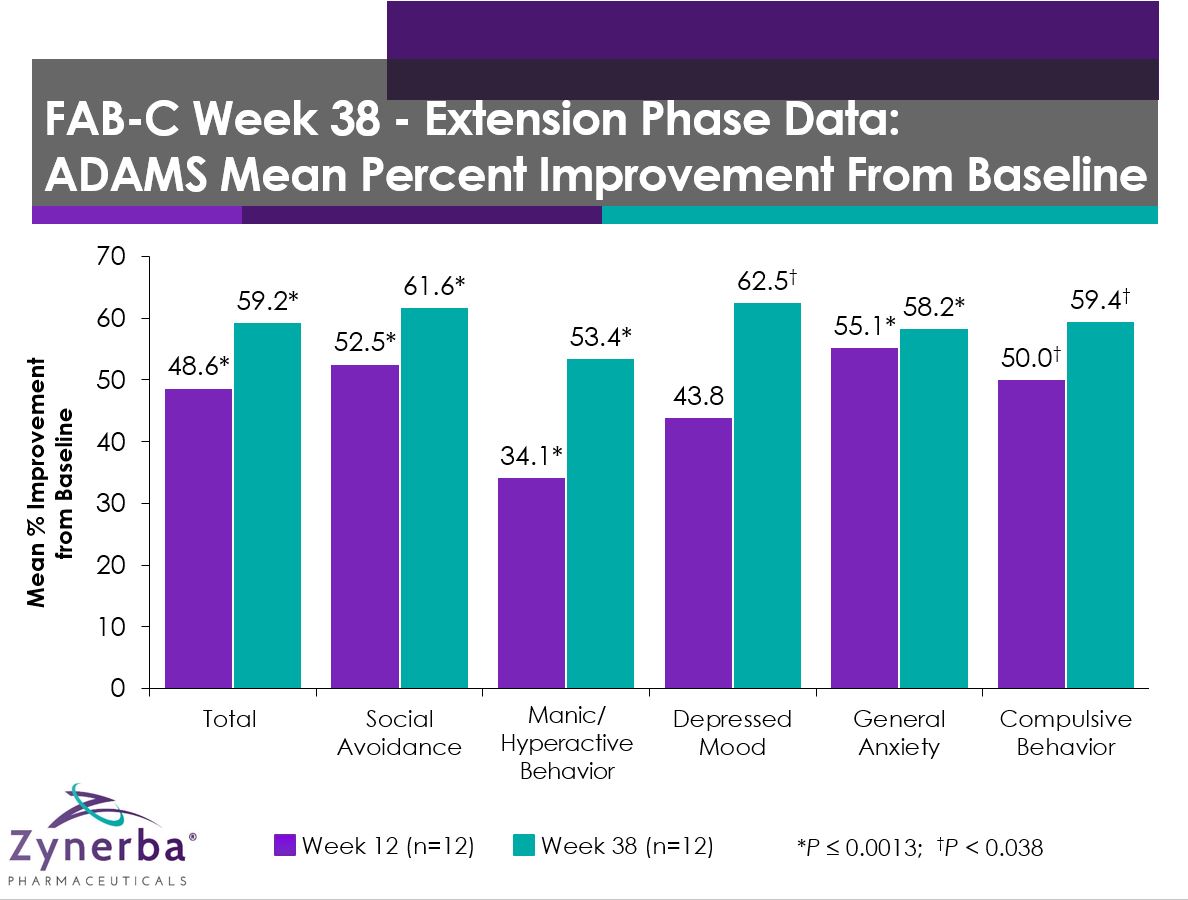
Long Term Efficacy: 12 and 38 Week
The following data show the improvement in various efficacy measures for the patients who completed 12 weeks, enrolled in the extension trial, and have completed 38 weeks of treatment.
Anxiety, Depression, and Mood Scale (ADAMS)
| Scale: ADAMS |
Group Mean Percent Improvement from Baseline |
| Week 12
(n=12) |
P-value vs Baseline |
Week 38
(n=12) |
P-value vs Baseline |
| ADAMS Total Score |
48.6 |
0.0001 |
59.2 |
<0.0001 |
| General Anxiety |
55.1 |
<0.0001 |
58.2 |
<0.0001 |
| Social Avoidance |
52.5 |
0.0013 |
61.6 |
0.0007 |
| Compulsive Behavior |
50.0 |
0.0295 |
59.4 |
0.0247 |
| Manic/Hyperactive Behavior |
34.1 |
0.0012 |
53.4 |
0.0002 |
| Depressed Mood |
43.8 |
0.0831 |
62.5 |
0.0372 |
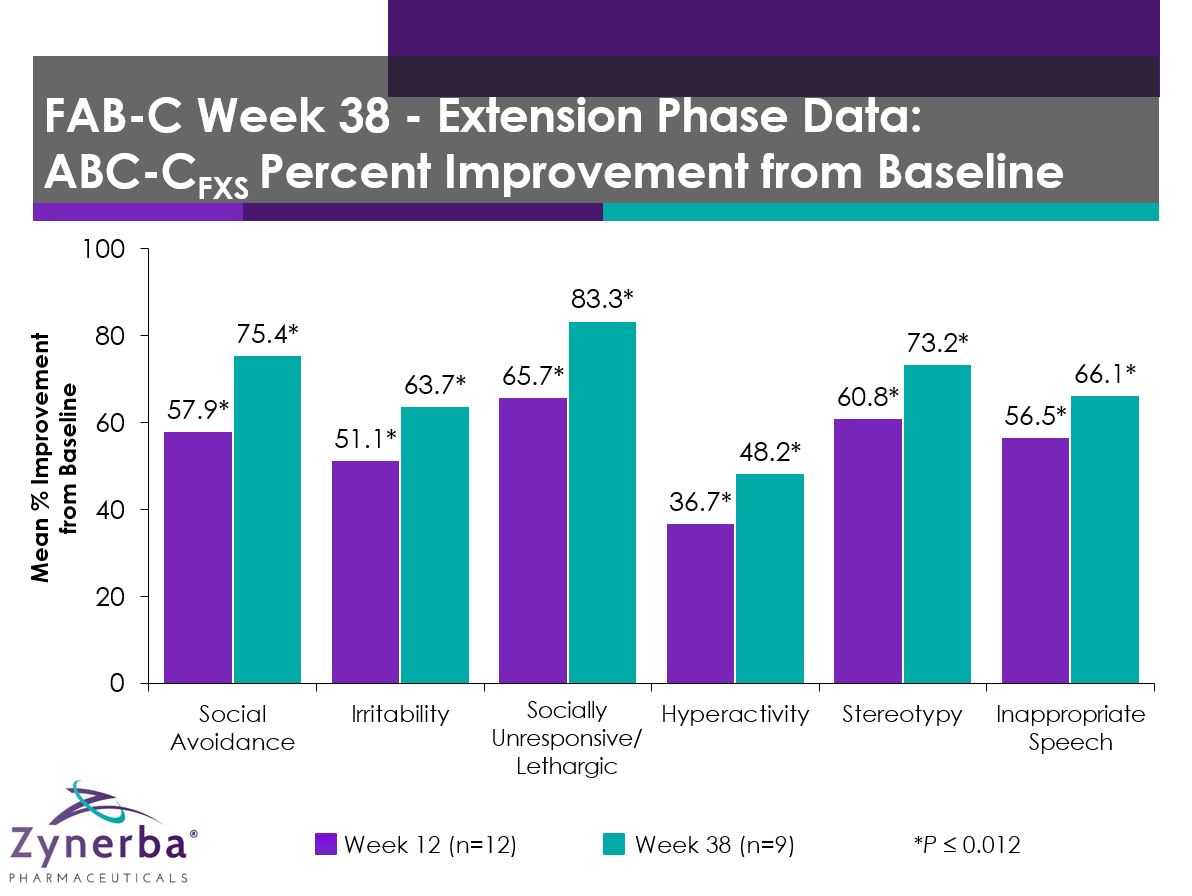
Aberrant Behavior Checklist – Community: FXS Specific (ABC-CFXS)
| Scale: ABC-CFXS |
Group Mean Percent Improvement from Baseline |
| Week 12
(n=12) |
P-value vs Baseline |
Week 38
(n=9) |
P-value vs Baseline |
| Stereotypy |
60.8 |
0.0048 |
73.2 |
0.0019 |
| Social Avoidance |
57.9 |
0.0040 |
75.4 |
0.0013 |
| Socially Unresponsive/Lethargic |
65.7 |
0.0024 |
83.3 |
0.0016 |
| Inappropriate Speech |
56.5 |
0.0002 |
66.1 |
<0.0001 |
| Irritability |
51.1 |
0.0012 |
63.7 |
0.0003 |
| Hyperactivity |
36.7 |
0.0119 |
48.2 |
0.0012 |
Safety Summary
ZYN002 was well tolerated, and the safety profile was consistent with previously reported clinical data, with no serious adverse events (SAEs) reported. Forty-three treatment-emergent adverse events (TEAEs) have been reported through week 38, all of which were mild or moderate. Most were unrelated to treatment with ZYN002. The most common TEAEs were gastroenteritis (14%) and upper respiratory tract infections (12%); all were considered unrelated and resolved during the study period. One patient, who has continued in the trial, developed moderate application rash, which resolved. No THC was detected in the plasma. No clinically meaningful trends in vital signs, ECG, or clinical safety laboratories including liver function tests (LFTs) were observed.
The Company plans to seek publication of these data and will provide future updates on the ongoing FAB-C study.
About Fragile X syndrome (FXS)
Fragile X syndrome is a rare genetic developmental disability that is the leading known cause of both inherited intellectual disability and autism spectrum disorder, affecting 1 in 3,600 to 4,000 males and 1 in 4,000 to 6,000 females. It is the most common inherited intellectual disability in males and a significant cause of intellectual disability in females. It is caused by a mutation in the Fragile X Mental Retardation gene (FMR1) located on the X chromosome and leads to dysregulation of the endocannabinoid pathway including the reduction in endogenous cannabinoids (2-AG and anandamide). The disorder negatively affects synaptic function, plasticity and neuronal connections, and results in a spectrum of intellectual disabilities and behavioral symptoms, such as social avoidance and irritability. In the US, there are about 71,000 patients suffering with FXS.
About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals is the leader in pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders. We are committed to improving the lives of patients and their families living with severe, chronic health conditions including Fragile X syndrome and refractory epilepsies. Learn more at www.zynerba.com and follow us on Twitter at @ZynerbaPharma
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company’s ability to obtain additional funding to support its clinical development programs; the results, cost and timing of the Company’s clinical development programs, including any delays to such clinical trials relating to enrollment or site initiation; clinical results for the Company’s product candidates may not be replicated or continue to occur in additional trials and may not otherwise support further development in a specified indication or at all; actions or advice of the U.S. Food and Drug Administration and foreign regulatory agencies may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company’s ability to obtain and maintain regulatory approval for its product candidates, and the labeling under any such approval; the Company’s reliance on third parties to assist in conducting pre-clinical and clinical trials for its product candidates; delays, interruptions or failures in the manufacture and supply of the Company’s product candidates the Company’s ability to commercialize its product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rate and degree of market acceptance of the Company’s product candidates; and the Company’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates. This list is not exhaustive and these and other risks are described in the Company’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission and available at www.sec.gov. Any forward-looking statements that the Company makes in this press release speak only as of the date of this press release. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
Investor Contact
Will Roberts, VP Investor Relations and Corporate Communications
Zynerba Pharmaceuticals
484.581.7489
robertsw@zynerba.com


