5 Nov 19
Zynerba Pharmaceuticals Reports Third Quarter 2019 Financial Results and Operational Highlights
– Topline Results from Pivotal Trial in Fragile X Syndrome On Track for 1H2020 –
– Positive Topline Results from BELIEVE 1 Phase 2 Trial of Zygel™ in Children and Adolescents with Developmental and Epileptic Encephalopathies Suggest Compelling Seizure Reductions and Excellent Tolerability –
– Phase 2 Topline Results in Autism Spectrum Disorder and 22q11.2 Deletion Syndrome On Track for 1H2020 –
Devon, PA, November 6, 2019 — Zynerba Pharmaceuticals, Inc. (NASDAQ:ZYNE), the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders, today reported financial results for the third quarter ended September 30, 2019 and provided an overview of recent operational highlights.
“The third quarter of 2019 was a remarkable period of progress and execution for Zynerba,” said Armando Anido, Chairman and Chief Executive Officer of Zynerba. “We announced compelling topline safety and efficacy results from our six month BELIEVE Phase 2 trial of Zygel™ in childhood epilepsies. In this study, patients experienced median reductions in their most common and debilitating seizures of 44% or more starting at month two and continuing through month six. In this medically fragile patient population, and consistent with our prior trials, Zygel was very well tolerated. Caregivers also reported important improvements in seizure intensity and duration in their children, and in socio-behavioral and cognitive impairments that are common in this population. Finally, we continued to progress towards full enrollment in our pivotal CONNECT-FX trial in children and adolescents with Fragile X syndrome, and expect to announce topline results in the first half of next year.”
Third Quarter 2019 Highlights
Zygel in Developmental and Epileptic Encephalopathies (DEE)
Presented Positive Topline Efficacy and Tolerability Results from BELIEVE 1 Open Label Phase 2 DEE Study
The topline results of this six month Phase 2 evaluation of Zygel in 48 children and adolescents with various DEEs showed meaningful reductions in seizures and excellent tolerability. Patients experienced 44% to 58% monthly median reductions in focal impaired-awareness seizures (FIAS; previously known as complex partial seizures) and/or convulsive seizures (CS; focal to bilateral tonic-clonic seizures and generalized tonic-clonic seizures), the most common and debilitating seizure types, starting at month two and continuing through month six. In addition, 42% to 63% of patients experienced a ≥50% monthly reduction in these seizures. Children with DEE are medically fragile, and as such, adverse events (all events, whether unrelated or related to study drug, that occur during the trial period) are common and expected. Only ten patients experienced a serious adverse event (SAE). Of those ten, eight were deemed to be unrelated to drug and only two were deemed possibly related to study drug, including one case of lower respiratory tract infection and one case of status epilepticus, both of which are common events in this patient population. There were no drug-related hepatic, gastrointestinal, or lethargy-related SAEs observed during this study.
Presented Qualitative Data Evaluating the Impact of Zygel on Quality of Life of Children with DEE
As part of the BELIEVE 1 study, caregivers were asked to provide a qualitative assessment regarding their child’s overall experiences during treatment with Zygel. Caregiver feedback to a series of open-ended questions was collected and coded by two independent reviewers. These qualitative assessments indicated improvements in alertness, awareness, or energy (58% of caregivers); seizures (51% of caregivers); cognition/concentration (47% of caregivers); socially-avoidant behaviors (44% of caregivers); and school attendance (28% of caregivers).
Preparations Underway for First Half 2020 Meeting with U.S. Food and Drug Administration (FDA) to Discuss Pathway for Zygel in DEE
Zynerba intends to meet with the FDA in the first half of 2020 to discuss the clinical path forward in DEE. Based on the Phase 2 trial design and results, the Company anticipates that it will discuss the pursuit of an indication that includes all syndromes and encephalopathies in the DEE category that present with FIAS and/or CS, the most common and debilitating seizure types representing 75% to 80% of all seizures.
Zygel in Fragile X Syndrome (FXS)
Fragile X Syndrome Pivotal Data Expected in the First Half of 2020
Enrollment is progressing in CONNECT-FX, a pivotal, multi-national, randomized, double blind, placebo-controlled trial evaluating the efficacy and safety of Zygel in treating common behavioral symptoms of FXS in three through 17-year old patients with FXS. The Company expects to report top line results in the first half of 2020. The primary endpoint is the change from baseline to the end of the treatment period in the Aberrant Behavior Checklist-Community FXS Specific (ABC-CFXS) Social Avoidance subscale. Key secondary endpoints are the change from baseline to the end of the treatment period in the ABC-CFXS Irritability subscale score, the ABC-CFXS Socially Unresponsive/Lethargic subscale score, and improvement in Clinical Global Impression – Improvement (CGI-I) at the end of the treatment period. If the results are positive, the Company expects to submit its New Drug Application (NDA) for Zygel in FXS to the U.S. Food and Drug Administration (FDA) in the second half of 2020, with potential approval by mid-year 2021.
Poster Further Validating the Use of the ABC-CFXS Presented at the 22nd Society for the Study of Behavioural Phenotypes (SSBP) Symposium
The poster described data collected via web-based journals and in-depth interviews of caregivers of children with FXS. The data indicate that nine of ten caregivers (90%) reported that their children had behaviors representative of social avoidance, socially unresponsiveness/lethargic, and irritability and that the described behaviors had strong concordance with individual domains of the ABC-CFXS. We believe that these data help elucidate the most common core behaviors of FXS, and further validate the appropriateness of the ABC-CFXS as an effective tool for use in clinical studies as a means to measure improvements in these core and common FXS behaviors.
Zygel 12-week Open Label Phase 2 FXS Data Published in the Journal of Neurodevelopmental Disorders
The results of the Phase 2 FAB-C clinical trial have been published in the peer-reviewed Journal of Neurodevelopmental Disorders in a paper entitled, ‘A Phase 1/2, Open Label Assessment of the Safety, Tolerability, and Efficacy of Transdermal Cannabidiol (ZYN002) for the Treatment of Pediatric Fragile X Syndrome’ (Heussler, Helen; Cohen, Jonathan; Silove, Natalie, et al.).
Zygel in Autism Spectrum Disorder (ASD)
Phase 2 Open Label Trial of Zygel in ASD Ongoing; Data Expected in the First Half of 2020
The Company is conducting the Phase 2 BRIGHT trial to assess the safety, tolerability and efficacy of Zygel for the treatment of child and adolescent patients with ASD. The 14-week trial is designed to evaluate the efficacy and safety of Zygel in approximately 36 children and adolescents (ages four through 17) with ASD as confirmed by DSM-5 diagnostic criteria for ASD. The efficacy assessments include the Aberrant Behavior Checklist, Parent Rated Anxiety Scale – Autism Spectrum Disorder, Autism Impact Measure, and Clinical Global Impression – Severity and Improvement. Zynerba expects to report topline results from this study in the first half of 2020.
Poster Describing the Shared Sociobehavioral Symptoms in ASD, FXS, and 22q11.2 Deletion Syndrome Presented at the 22nd SSBP Symposium
The poster described the results of a retrospective literature review on patients with ASD, FXS, and 22q11.2 deletion syndrome (22q) conducted to determine symptomatic overlap between these disorders. The data indicate that patients with ASD, FXS, and 22q share a constellation of sociobehavioral symptoms and that the pharmacology of CBD is broad, continues to be defined, and may prove to be beneficial in addressing important behavioral symptoms of these conditions.
Zygel in 22q11.2 Deletion Syndrome (22q)
Phase 2 Open Label Trial of Zygel in 22q Ongoing; Data Expected in the First Half of 2020
The Company is conducting the 14-week Phase 2 INSPIRE trial to evaluate the safety, tolerability and efficacy of Zygel in approximately 20 children and adolescents (ages six through 17) with genetically-confirmed 22q. The efficacy assessments include the Aberrant Behavior Checklist-Community (ABC-C), the Anxiety, Depression and Mood Scale (ADAMS), the Qualitative Caregiver Reported Behavioral Problem Survey, and Clinical Global Impression – Severity and Improvement. Zynerba expects to report topline results from this study in the first half of 2020.
Third quarter 2019 Financial Results
Our Australian subsidiary, Zynerba Pharmaceuticals Pty Ltd, or the Subsidiary, is incorporated in Australia and is eligible to participate in an Australian research and development tax incentive program. As part of this program, the Subsidiary is eligible to receive a cash refund from the Australian Taxation Office for a percentage of the research and development costs expended by the Subsidiary in Australia. In July 2019, the Australian government’s Department of Industry, Innovation and Science, or AusIndustry, responded to an Advance Overseas Finding, or AOF, application submitted by Zynerba that will allow certain research and development expenses incurred with respect to our product candidate Zygel outside of Australia to be eligible for the Australian research and development tax incentive program. As a result of this finding, we are eligible to receive a cash refund from the Australian Taxation Office for the qualifying research and development costs expended outside of Australia in 2018, 2019 and 2020. During the three months ending September 30, 2019, we recorded an $8.3 million credit to research and development expenses for amounts expected to be received through the AOF for the period from January 1, 2018 through September 30, 2019. Although the AOF approval extends into 2020, management believes that substantially all qualifying amounts have been recorded as of September 30, 2019.
The following table summarizes research and development expenses for the three months ended September 30, 2019 and 2018.
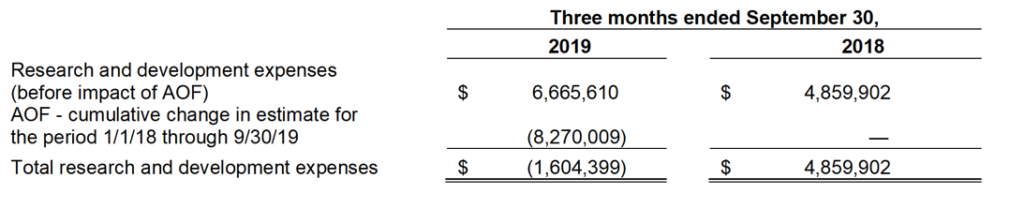
Excluding the $8.3 million reduction in research and development expenses for amounts expected to be received through the AOF for the period from January 1, 2018 through September 30, 2019, research and development expenses increased by $1.8 million to $6.7 million for the three months ended September 30, 2019 from $4.9 million for the three months ended September 30, 2018. The increase was primarily related to an increase in clinical trial and manufacturing costs related to our Zygel program. Stock based compensation included in the R&D costs were $0.6 million.
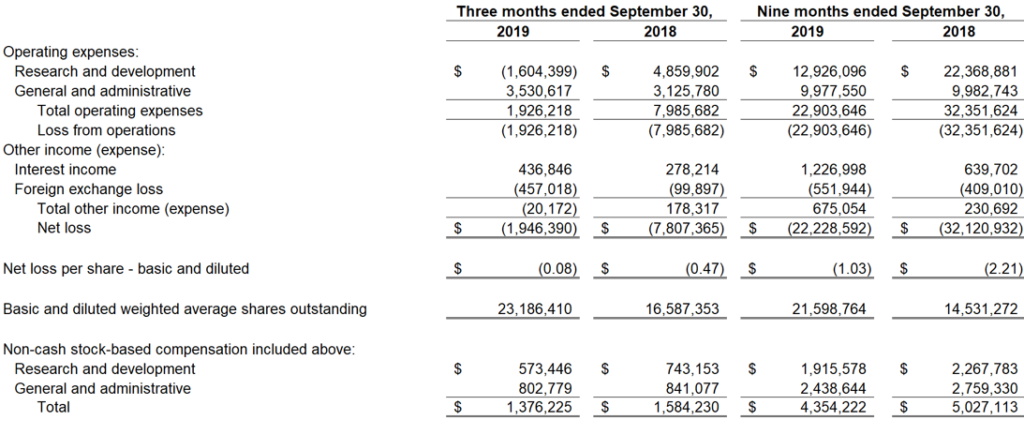
General and administrative expenses for the third quarter of 2019 were $3.5 million, including stock-based compensation expense of $0.8 million.
The net loss for the third quarter of 2019 was $1.9 million with basic and diluted net loss per share of $(0.08).
Financial Outlook
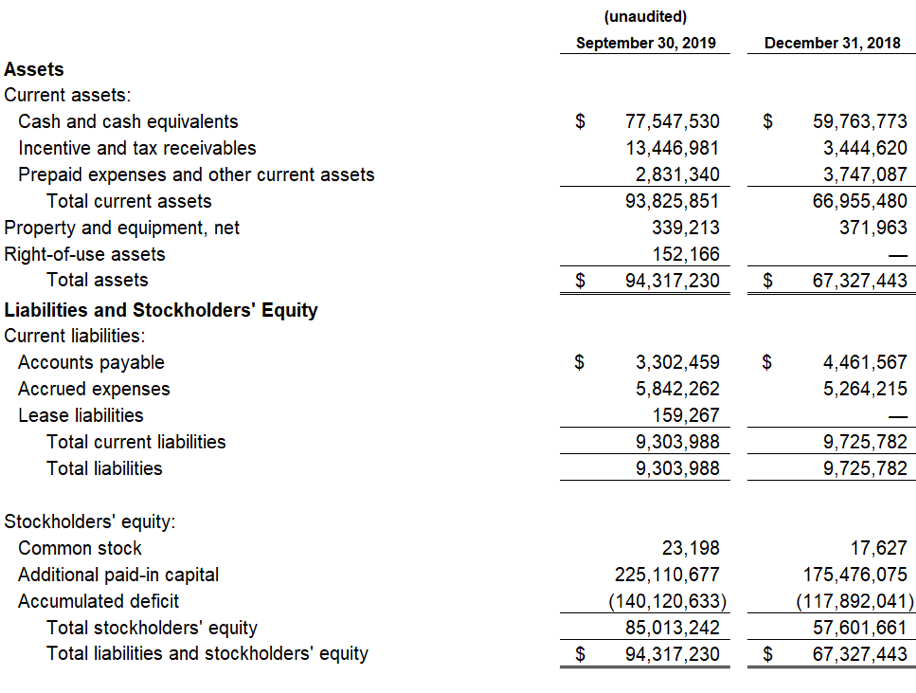
The Company’s cash and cash equivalent position as of September 30, 2019 was $77.5 million. Management believes that the cash and cash equivalent position is sufficient to fund operations and capital requirements beyond the expected NDA submission and potential approval of Zygel in FXS and into the second half of 2021.
About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals is the leader in pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders. We are committed to improving the lives of patients and their families living with severe, chronic health conditions including Fragile X syndrome, autism spectrum disorder, 22q11.2 deletion syndrome, and a heterogeneous group of rare and ultra-rare epilepsies known as developmental and epileptic encephalopathies. Learn more at www.zynerba.com and follow us on Twitter at @ZynerbaPharma.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements
within the meaning of The Private Securities Litigation Reform Act of 1995. We
may, in some cases, use terms such as “predicts,” “believes,” “potential,”
“proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,”
“intends,” “may,” “could,” “might,” “will,” “should” or other words that convey
uncertainty of future events or outcomes to identify these forward-looking statements.
Such statements are subject to numerous important factors, risks and
uncertainties that may cause actual events or results to differ materially from
the Company’s current expectations. Management’s expectations and, therefore,
any forward-looking statements in this press release could also be affected by
risks and uncertainties relating to a number of other factors, including the
following: the Company’s cash and cash equivalents may not be sufficient to
support its operating plan for as long as anticipated; the Company’s ability to
obtain additional funding to support its clinical development programs; the
results, cost and timing of the Company’s clinical development programs,
including any delays to such clinical trials relating to enrollment or site
initiation; clinical results for the Company’s product candidates may not be
replicated or continue to occur in additional trials and may not otherwise
support further development in a specified indication or at all; actions or
advice of the U.S. Food and Drug Administration and foreign regulatory agencies
may affect the design, initiation, timing, continuation and/or progress of
clinical trials or result in the need for additional clinical trials; the
Company’s ability to obtain and maintain regulatory approval for its product
candidates, and the labeling under any such approval; the Company’s reliance on
third parties to assist in conducting pre-clinical and clinical trials for its
product candidates; delays, interruptions or failures in the manufacture and
supply of the Company’s product candidates the Company’s ability to
commercialize its product candidates; the size and growth potential of the
markets for the Company’s product candidates, and the Company’s ability to
service those markets; the Company’s ability to develop sales and marketing
capabilities, whether alone or with potential future collaborators; the rate
and degree of market acceptance of the Company’s product candidates; and the
Company’s expectations regarding its ability to obtain and adequately maintain
sufficient intellectual property protection for its product candidates. This
list is not exhaustive and these and other risks are described in the Company’s
periodic reports, including the annual report on Form 10-K, quarterly reports
on Form 10-Q and current reports on Form 8-K, filed with or furnished to the
Securities and Exchange Commission and available at www.sec.gov. Any
forward-looking statements that the Company makes in this press release speak
only as of the date of this press release. The Company assumes no obligation to
update forward-looking statements whether as a result of new information,
future events or otherwise, after the date of this press release.
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
Zynerba Contacts
Jim Fickenscher, CFO and VP Corporate Development
Zynerba Pharmaceuticals
484.581.7483
fickenscherj@zynerba.com
Will Roberts, VP Investor Relations and Corporate CommunicationsZynerba Pharmaceuticals
484.581.7489
robertsw@zynerba.com

