8 May 19
Zynerba Pharmaceuticals Reports First Quarter 2019 Financial Results and Operational Highlights
Devon, PA, May 8, 2019 — Zynerba Pharmaceuticals, Inc. (NASDAQ:ZYNE), the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders, today reported financial results for the first quarter ended March 31, 2019 and provided an overview of recent operational highlights.
“We are entering a transformational period for Zynerba during which we expect top line data from four neuropsychiatric disorder trials with Zygel™, our patent protected CBD gel,” said Armando Anido, Chairman and Chief Executive Officer of Zynerba. “We’ve made great progress with Zygel which was recently designated as a Fast Track development program by the FDA for treating behavioral symptoms of Fragile X Syndrome. Over the next 14 months, we expect to announce data from the CONNECT-FX pivotal trial in Fragile X, our Phase 2 BELIEVE 1 trial in developmental and epileptic encephalopathies, our Phase 2 BRIGHT trial in Autism Spectrum Disorder, and the Phase 2 trial in 22q11.2 Deletion Syndrome that we intend to initiate this quarter. We expect that our cash position will take us through all of these data readouts, through our anticipated NDA submission and potential approval for Zygel in FXS, and into the first quarter of 2021.”
First Quarter 2019 and Recent Highlights
Zygel in Fragile X Syndrome (FXS)
Fragile X Syndrome Pivotal Data Expected in the Second Half of 2019
Enrollment is progressing in CONNECT-FX, a pivotal, multi-national, randomized, double blind, placebo-controlled trial evaluating the efficacy and safety of Zygel (ZYN002 CBD gel) in three through 17-year old patients with FXS. The primary endpoint is the change from baseline to the end of the treatment period in the Aberrant Behavior Checklist-Community FXS Specific (ABC-CFXS) Social Avoidance subscale. Clinical investigative sites are enrolling patients in the United States, Australia, and New Zealand. Patients who have completed the double-blind phase are now enrolling into the 12-month open label extension phase. The Company expects to report top line data in the second half of 2019. If the data are positive, the Company expects to submit its New Drug Application (NDA) for Zygel in FXS to the U.S. Food and Drug Administration(FDA) in the first half of 2020, with potential approval by year-end 2020.
Received Fast Track Designation for Zygel for Treatment of Behavioral Symptoms Associated with Fragile X Syndrome
FDA’s Fast Track program is designed to facilitate the development of drugs intended to treat serious conditions and fill unmet medical needs and can lead to expedited review by FDA in order to get new important drugs to the patient earlier.
Announced Receipt of New U.S. Patent for Treatment of Fragile X Syndrome with Cannabidiol (CBD)
The U.S. Patent and Trademark Office issued U.S. Patent No. 10,213,390 titled “Treatment of Fragile X Syndrome with Cannabidiol” which includes claims directed to methods of treating FXS by administering a therapeutically effective amount of synthetic or purified CBD. This new patent expires in 2038 and is part of an expanding intellectual property portfolio covering Zygel.
Zygel in Developmental and Epileptic Encephalopathies (DEE)
Topline Results Expected in the Third Quarter of 2019
Enrollment is complete in BELIEVE 1, an open label multi-dose Phase 2 clinical trial evaluating the efficacy and safety of Zygel in children and adolescents (three through 17 years) with DEE. The primary efficacy assessment is reduction in seizure frequency at week 26 compared to baseline. The Company expects to announce topline data through week 26 in the third quarter of 2019.
Zygel in Autism Spectrum Disorder (ASD)
Announced Initiation of Phase 2 Open Label Study in Zygel in ASD; Data Expected in the First Half of 2020
The Company initiated the Phase 2 BRIGHT trial in March 2019 to assess the safety, tolerability and efficacy of Zygel for the treatment of child and adolescent patients with ASD. The 14-week trial is designed to evaluate the efficacy and safety of Zygel in approximately 36 children and adolescents (ages four through 17) with ASD as confirmed by DSM-5 diagnostic criteria for ASD. The efficacy assessments include the Aberrant Behavior Checklist, Parent Rated Anxiety Scale – Autism Spectrum Disorder, Autism Impact Measure, and Clinical Global Impression – Severity and Improvement. Zynerba expects to present topline data from this study in the first half of 2020.
Zygel in 22q11.2 Deletion Syndrome (22q)
Initiation of Phase 2 Trial in 22q Anticipated Later this Quarter; Data Expected in the First Half of 2020
Zynerba has finalized the protocol to evaluate the efficacy and safety of Zygel in approximately 20 children and adolescents (ages six through 17) with genetically-confirmed 22q. The Company expects to initiate this study during the second quarter of 2019, and to present topline data in the first half of 2020.
Corporate
Dr. Thomas Harrison resigned from the Company’s Board of Directors to focus on his role with Merida Capital Partners as Senior Operating Partner. Dr. Harrison served on Zynerba’s Board of Directors since April 2015.
“On behalf of Zynerba’s Board of Directors and management team, I would like to thank Tom for his outstanding service and leadership during his term as a member of our board,” continued Mr. Anido. “His expertise and counsel have been invaluable to Zynerba and we wish him well in his new endeavor.”
First Quarter 2019 Financial Results
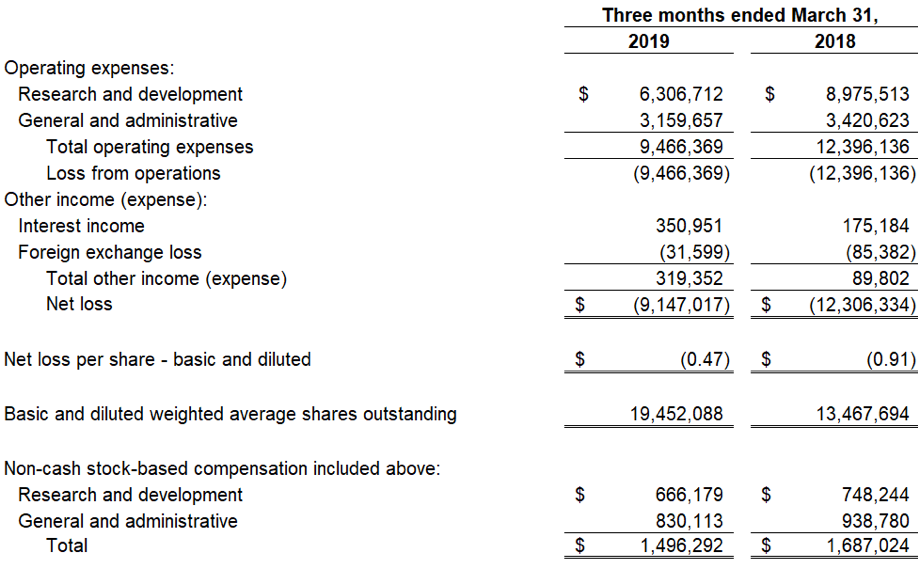
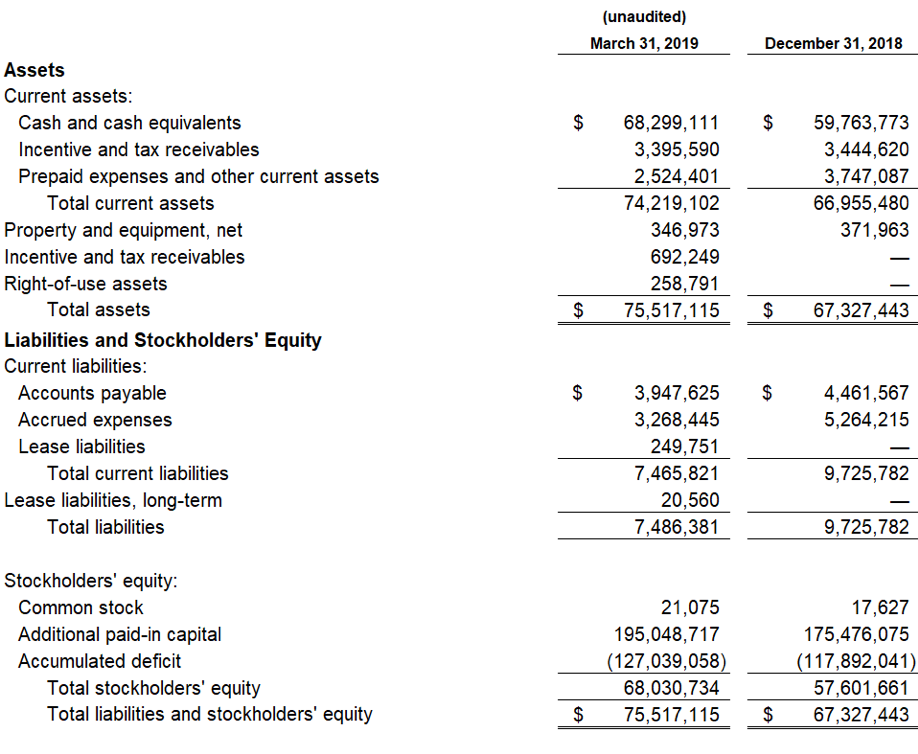
As of March 31, 2019, cash and cash equivalents were $68.3 million, compared to $59.8 million as of December 31, 2018. Included in this cash and cash equivalents position is $18.1 million in net proceeds from the 3,439,523 shares sold and issued at a weighted average selling price of $5.44 per share during the first quarter of 2019 under an Open Market Sales Agreement, or “at-the-market” (ATM) offering program, with Jefferies LLC. Research and development expenses for the first quarter of 2019 were $6.3 million, including stock-based compensation of $0.7 million. General and administrative expenses for the first quarter of 2019 were $3.2 million, including stock-based compensation expense of $0.8 million. The net loss for the first quarter of 2019 was $9.1 million with basic and diluted net loss per share of $(0.47).
Financial Outlook
The Company’s cash and cash equivalent position as of March 31, 2019 was $68.3 million. Management believes that the cash and cash equivalent position is sufficient to fund operations and capital requirements beyond the expected NDA submission and potential approval in FXS and into the first quarter of 2021.
About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals is the leader in pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders. We are committed to improving the lives of patients and their families living with severe, chronic health conditions including Fragile X Syndrome, Autism Spectrum Disorder, 22q11.2 Deletion Syndrome, and a heterogeneous group of rare and ultra-rare epilepsies known as developmental and epileptic encephalopathies. Learn more at www.zynerba.com and follow us on Twitter at @ZynerbaPharma.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company’s ability to obtain additional funding to support its clinical development programs; the results, cost and timing of the Company’s clinical development programs, including any delays to such clinical trials relating to enrollment or site initiation; clinical results for the Company’s product candidates may not be replicated or continue to occur in additional trials and may not otherwise support further development in a specified indication or at all; actions or advice of the U.S. Food and Drug Administrationand foreign regulatory agencies may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company’s ability to obtain and maintain regulatory approval for its product candidates, and the labeling under any such approval; the Company’s reliance on third parties to assist in conducting pre-clinical and clinical trials for its product candidates; delays, interruptions or failures in the manufacture and supply of the Company’s product candidates the Company’s ability to commercialize its product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rate and degree of market acceptance of the Company’s product candidates; and the Company’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates. This list is not exhaustive and these and other risks are described in the Company’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commissionand available at www.sec.gov. Any forward-looking statements that the Company makes in this press release speak only as of the date of this press release. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
Zynerba Contacts
Jim Fickenscher, CFO and VP Corporate Development
Zynerba Pharmaceuticals
484.581.7483
fickenscherj@zynerba.com
Will Roberts, VP Investor Relations and Corporate Communications
Zynerba Pharmaceuticals
484.581.7489
robertsw@zynerba.com